Search This Supplers Products:GlasswareGlass Coffee SetGlass CupGlass TeapotGlass jarGlass Perfume Bottle
The process of glass making
The process of glass making
When US scientists tested a prototype of the atomic bomb in the New Mexico desert in 1945, the explosion turned the sand in the immediate area of the impact into glass. Fortunately, there are easier and less extreme ways of making glass—but all of them need immense amounts of heat.
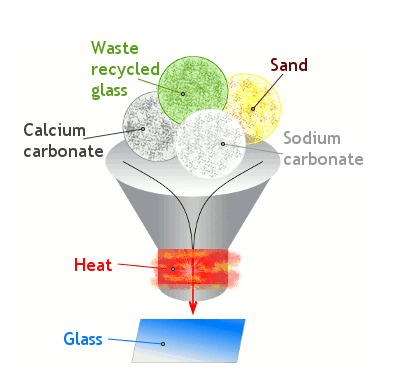
In a commercial glass plant, sand is mixed with waste glass (from recycling collections), soda ash (sodium carbonate), and limestone (calcium carbonate) and heated in a furnace. The soda reduces the sand's melting point, which helps to save energy during manufacture, but it has an unfortunate drawback: it produces a kind of glass that would dissolve in water! The limestone is added to stop that happening. The end-product is called soda-lime-silica glass. It's the ordinary glass we can see all around us.
Borosilicate glass, such as this PYREX,jug (back), can withstand extreme changes of temperature, unlike normal glass (front), which shatters. The ordinary glass jar at the front is quite a bit thinner and considerably lighter. You can also see, very clearly that the borosilicate glass is a slightly blueish color (as is the boron oxide from which it's made).
Once the sand is melted, it is either poured into molds to make bottles, glasses, and other containers, or "floated" (poured on top of a big vat of molten tin metal) to make perfectly flat sheets of glass for windows. Unusual glass containers are still sometimes made by "blowing" them. A "gob" (lump) of molten glass is wrapped around an open pipe, which is slowly rotated. Air is blown through the pipe's open end, causing the glass to blow up like a balloon. With skillful blowing and turning, all kinds of amazing shapes can be made.
Glass makers use a slightly different process depending on the type of glass they want to make. Usually, other chemicals are added to change the appearance or properties of the finished glass. For example, iron and chromium based chemicals are added to the molten sand to make green-tinted glass. Oven-proof borosilicate glass (widely sold under the trademark PYREX?) is made by adding boron oxide to the molten mixture. Adding lead oxide makes a fine crystal glass that can be cut more easily; highly prized cut lead crystal sparkles with color as it refracts (bends) the light passing through it. Some special types of glass are made by a different manufacturing process. Bulletproof glass is made from a sandwich or laminate of multiple layers of glass and plastic bonded together. Toughened glass used in car windshields is made by cooling molten glass very quickly to make it much harder. Stained (colored) glass is made by adding metallic compounds to glass while it is molten; different metals give the separate segments of glass their different colors.
